|
|
Cell shape on micropatterned substrates
|
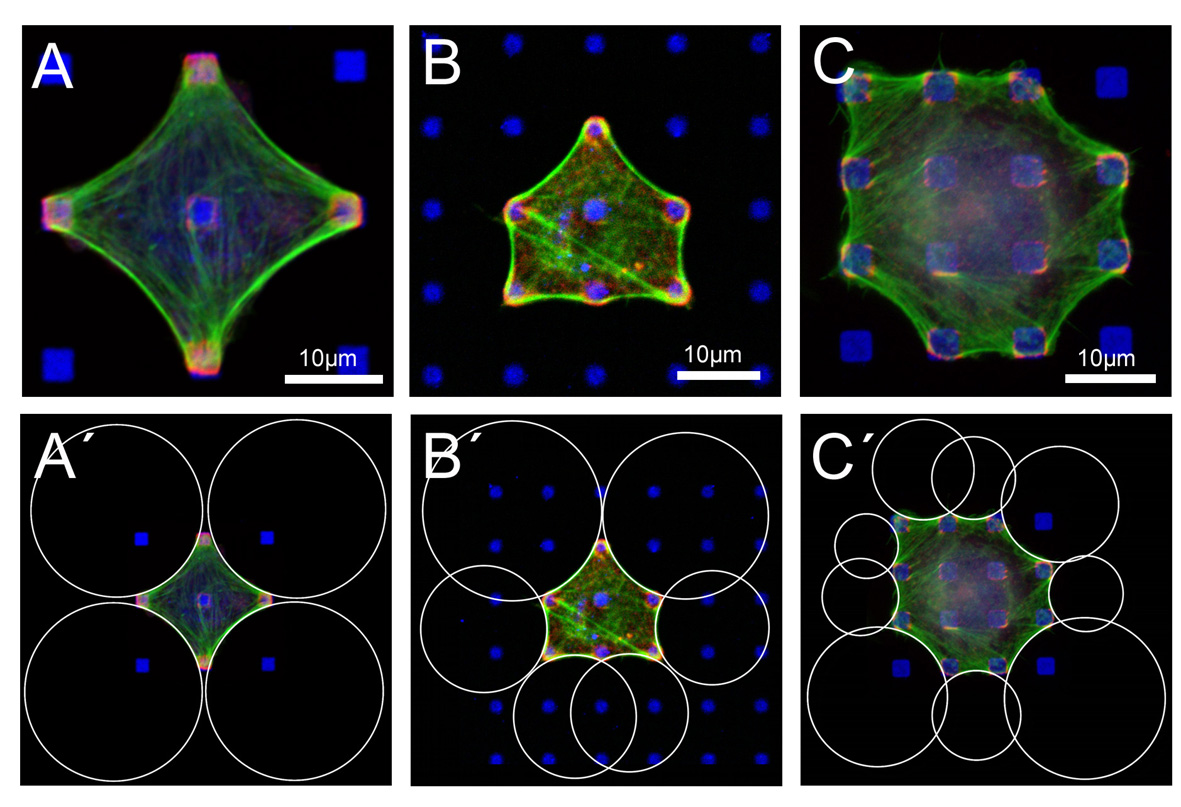
The contour of fibroblast-like cells adhering to micropatterned dot patterns shows a typical sequence of inward-direct circular arcs. In the upper row, BRL (A and B) and B16 (C) cells have been cultured on fibronectin dots (blue). Focal adhesions (paxillin, red) localize at these dots and actin (green) reenforces the contour. In the lower row (A'-C'), we have fitted circles to these circular arcs. This procedure works well if cell mechanics is not dominated by internal actin structures like stress fibers. It allows us to investigate cell shape as a function of extracellular matrix geometry. In particular, we have correlated the extracted radii with the spanning distance between two neighboring adhesions. We find that arc radius increases with spanning distance (note for example the larger circles at the diagonals in B'), which indicates that the Laplace law explaining the circular features has to be extended by elastic elements introducing absolute lengths. A computational analysis shows that the Laplace-type law can only be rescued by assuming the cell to act as an actively contracting cable network (assuming a Hookean network does not result in circular features). This makes sense because cell shape is known to be determined mainly be the filamenteous mechanics of the actin network. Here the contractile agents are mainly myosin motors distributed for example in the actin cortex underlying the plasma membrane and in the actin cables lining the cell contour. Surprisingly, the same shape features have been found for a simple tissue model (contractile cells in a collagen gel which is pinned at discrete sites to a flat substrate), indicating that the same principles (filamenteous network mechanics and active contractility) act both on cell and tissue shapes.
Publications:
- I. B. Bischofs, F. Klein, D. Lehnert, M. Bastmeyer, and U. S. Schwarz. Filamentous network mechanics and active contractility determine cell and tissue shape. Biophys. J., 95:3488-3496, 2008. (abstract, PDF)
- I. B. Bischofs, S. S. Schmidt, and U. S. Schwarz. Effect of adhesion geometry and rigidity on cellular force distributions. Phys. Rev. Lett., 103:048101, 2009. (abstract, q-bio arXiv:0907.4114, doi:10.1103/PhysRevLett.103.048101, PDF)
Last modified Di 4. Okt 17:45:17 CEST 2011
by USS.
Back to home page Ulrich Schwarz.