|
|
Cell capture and rolling in hydrodynamic flow
 |
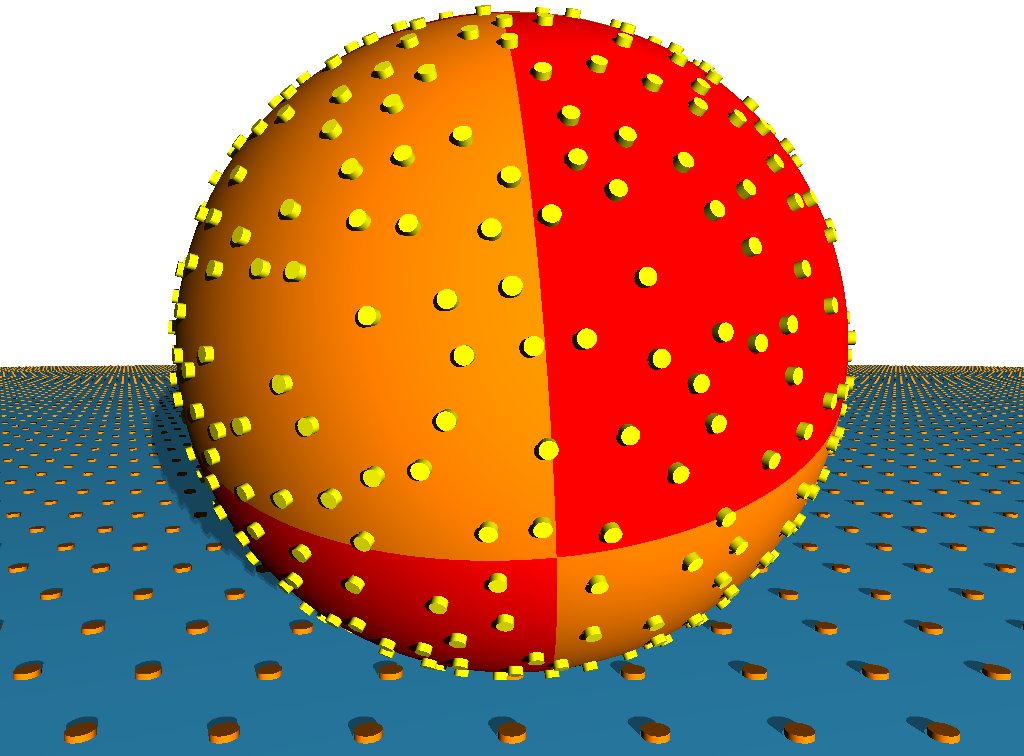 |
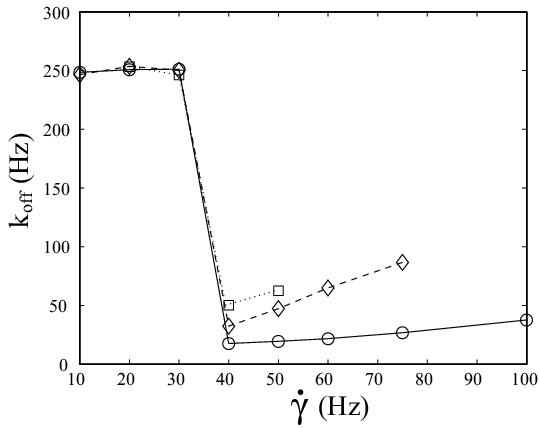
| L-selectin tether dissociation rate as function of shear rate as determined from kinetic analysis of flow chamber experiments with white blood cells. Solid line with circles: wildtype. Dashed line with diamonds: tail-deleted mutant. Dotted line with squares: wildtype with increased viscosity of the medium. Our quantitative modelling aims at explaining the effective dissociation kinetics observed experimentally in terms of molecular processes at the cell-substrate interface. Schwarz and Alon, PNAS 2004. | Adhesive dynamics simulations. Using a Langevin-equation approach for a sphere covered with reaction patches and moving in hydrodynamic flow above a wall, we simulate how cells attach to and move on ligand-coated substrates under flow conditions. Korn and Schwarz, PRL 2006. |
Leukocyte are white blood cells and they permanently travel our body with the blood stream. Because their biological function is to locate and fight pathogens, they have to exit the blood stream into the surrounding tissue. This they do through a complicated process called the extravasation cascade. The most prominent sites of leukocyte extravasation are sites of infection and tissue injury as well as the spleen and the lymph nodes (it has been estimated that 1.4x104 leukocytes extravasate every second in every lymph node). The same mechanisms to travel our body are used also by stem and cancer cells. A failure of proper leukocyte adhesion can result in disease, as exemplified by the leukocyte-adhesion deficiency (LAD), which is caused by genetic lack of a certain functional integrin subunit. LAD leads to the inability of leukocytes to exit the blood stream and to migrate into open wounds.
The extravasation cascade of leukocytes starts with L-selectin mediated capture of cells from the blood flow. Since L-selectin has a fast dissociation rate, which is further increased by the force resulting from shear flow, cell capture is only transient. However, at sufficiently high ligand density, the dissociating cell is likely to be captured again by new bonds downstream of the initial bond. This sequence of events leads to rolling adhesion. In contrast to a macroscopic sticky sphere rolling down an inclined plane, rolling adhesion of leukocytes is based on discret molecular events and therefore leads to jerky movement. Rolling adhesion allows the cell to survey the blood vessel wall for signs of inflammation. One part of the inflammatory response is the presence of small molecules called cytokines on the endothelium. Cytokines signal through G-protein coupled receptors and activate stronger adhesion through integrins. After cell arrest, leukocytes start to migrate on the endothelium, finally undergoing transendothelial migration. The combination of selectins, cytokines and integrins results in a sophisticated zip-code systems which guarantees that different cell types exit exactly at those places where they are needed. In the tissue, leukocytes begin their hunt for pathogens, based on chemotactic movement guided by chemokines.
The most convenient tool to study the extravasation cascade is the parallel plate flow chamber, which allows to control flow conditions and ligand presentation. A lot of experimental effort has been directed towards studying the initial stages, namely cell capture through L-selectin. L-selectin is a leukocyte-expressed adhesion receptor which is localized to tips of leukocyte microvilli and which binds to glycosylated ligands on the endothelium (the major L-selectin ligand being PNAd). On highly diluted ligand, one can investigate the quantal unit of L-selectin meditated leukocyte rolling, namely single tethers. In the mid-90s, the first studies of this kind introduced the kinetic analysis of video data, which resulted in first-order dissociation kinetics and a shear-dependence of the dissociation rate which agreed well with the so-called Bell equation, which describes the forced dissociation of simple single bonds. Since then, flow chamber tethering to diluted ligand has been interpreted as single molecule experiment.
However, recent experimental evidence and theoretical modelling suggest that L-selectin mediated tethering is a more complicated process, possibly involving multiple bonds and many local rebinding events. In particular, our new findings suggest that L-selectin is specialized for initial cell capture in several regards: it seems to have an exceptionally fast dissociation rate, high strength under loading, and a fast rebinding rate. A long standing puzzle in L-selectin mediated tethering has been the so-called shear threshold phenomenon: in contrast to other receptors systems like P-selectin (expressed on platelets), E-selectin (expressed on endothelium) and integrins, L-selectin tethering and subsequent rolling only occurs above a threshold in shear, even in cell-free systems. Downregulation by low shear is unique for L-selectin tethers and might be necessary because L-selectin ligands are constitutively expressed on circulating leukocytes, platelets and subsets of blood vessel walls. In cooperation with the lab of Ronen Alon at the Weizmann Institute in Israel, we have shown both experimentally and theoretically that the notion of multiple bonds and fast rebinding can consistently explain the main features of L-selectin mediated leukocyte tethering in shear flow, namely effective first order dissociation, the Bell-like dependence on shear force and the shear threshold. An alternative explanation is that single L-selectin bonds act as catch bonds, which live longer under increased force.
The left figure above summarizes the most important experimental data. As it is measured with fast video camera (2 ms resolution versus 30 ms resolution in earlier work), the wildtype data (circles) shows that L-selectin tethers form even below the shear threshold at shear rate 40 Hz, albeit with a very fast dissociation rate of 250 Hz, which is undetectable with regular camera. Thus the shear threshold results from insufficient tether stabilization at low shear, rather than from insufficient tether formation. At the shear threshold, tether lifetime is prolongued by a factor of 14. The wildtype data above the shear threshold agrees well with earlier results with regular camera, in particular it shows the Bell-like dependence on shear force. Upon addition of 6 percent of the non-toxic sugar Ficoll to the medium, viscosity is increased by a factor of 2.6 (squares). Therefore shear stress, but not shear rate increases by the same factor. At the shear threshold, this changes wildtype dissociation 3-fold, roughly as expected from the Bell equation. However, the left figures above shows that it does not change the location of the shear threshold. This suggests that the shear threshold results from shear-mediated transport, rather than from a force-dependent process (like overcoming a repulsive barrier for ligand-receptor binding). The most likely event promoted by shear-mediated transport is the formation of multiple bonds. The presence of multiple bonds then opens up the possibility for rebinding of ruptured bonds, since only if additional closed bonds can hold the cell, the spatial proximity required for rebinding is guaranteed. The left figure above also shows dissociation data for leukocytes transfected with a variant of L-selectin (diamonds). In this mutant, the tail-region, which usually mediates anchorage to the cytoskeleton, has been deleted. There are several possible explanations why tail-deletion leads to faster tether dissociation, but the most likely explanation is that L-selectin tail-deletion leads to faster lateral mobility, which in turn interferes with rebinding and tether stabilization.
Motivated by the collaboration with the Alon lab, we started to develop a computational framework to simulate the phenomena of cell capture and rolling in hydrodynamic flow in the computer. This line of research is known as "adhesive dynamics simulations" and we extended this field by resolving for the first time the spatial positions of both receptors and ligands. For this end it is crucial to include stochastic fluctuations, because otherwise the nanometer-sized molecules would not be able to find each other and bind. We therefore set up a Langevin equation which includes the effect of direct, steric, hydrodynamic and thermal forces. Within this framework, we were then able to address the question of efficiency of cell capture by frameing it as a first passage time problem. One of our most important results is that efficiency of cell capture is increased dramatically by increasing the height of the receptors over the cell surface. This explains why white blood cells in the blood flow adhere through receptor patches localized to the tips of microvilli, and why malaria-infected red blood cells form elevated receptor patches (knobs) (Physical Review Letters 2006). We also used our computational framework to characterize the onset of rolling adhesion, in particular the transition from slipping to rolling (Physical Review E 2008).
Publications with the Alon group:
- O. Dwir, D. A. Steeber, U. S. Schwarz, R. T. Camphausen, G. S. Kansas, T. F. Tedder and R. Alon, L-selectin dimerization enhances tether formation to properly spaced ligand, J. Biol. Chem. 277: 21130-9 (2002) (abstract, PDF)
- O. Dwir, A. Solomon, S. Mangan, G. S. Kansas, U. S. Schwarz and R. Alon, Avidity enhancement of L-selectin bonds by flow: shear-promoted rotation of leukocytes turn labile bonds into functional tethers, J. Cell. Biol. 163: 649-59 (2003) (abstract, PDF)
- U. S. Schwarz and R. Alon, L-selectin mediated leukocyte tethering in shear flow is controled by multiple contacts and cytoskeletal anchorage facilitating fast rebinding events, Proc. Natl. Acad. Sci. USA 101: 6940-6945 (2004) (abstract, DOI: 10.1073/pnas.0305822101, PDF, PDF Supplemental Material)
A Langevin equation-based approach to cell capture and rolling adhesion:
- C. Korn and U. S. Schwarz. Efficiency of initiating cell adhesion in hydrodynamic flow. Phys. Rev. Lett. 97:138103, 2006. (abstract, cond-mat/0609093, doi:10.1103/PhysRevLett.97.138103, PDF)
- C. B. Korn and U. S. Schwarz. Mean first passage times for bond formation for a Brownian particle in linear shear flow above a wall. J. Chem. Phys., 126:095103, 2007. (abstract, cond-mat/0610302v2, doi:10.1063/1.2464080, PDF)
- C. B. Korn and U. S. Schwarz. Mean encounter times for cell adhesion in hydrodynamic flow: Analytical progress by dimensional reduction. Europhys. Lett., 83:28007, 2008. (abstract, PDF)
- C. B. Korn and U. S. Schwarz. Dynamic states of cells adhering in shear flow: From slipping to rolling. Phys. Rev. E, 77:041904, 2008. (abstract, q-bio arXiv:0711.2227, doi:10.1103/PhysRevE.77.041904, PDF)
Last modified Mon May 4 15:05:35 CEST 2009
by USS.
Back to home page Ulrich Schwarz.