|
|
Virus uptake, assembly and spread
|
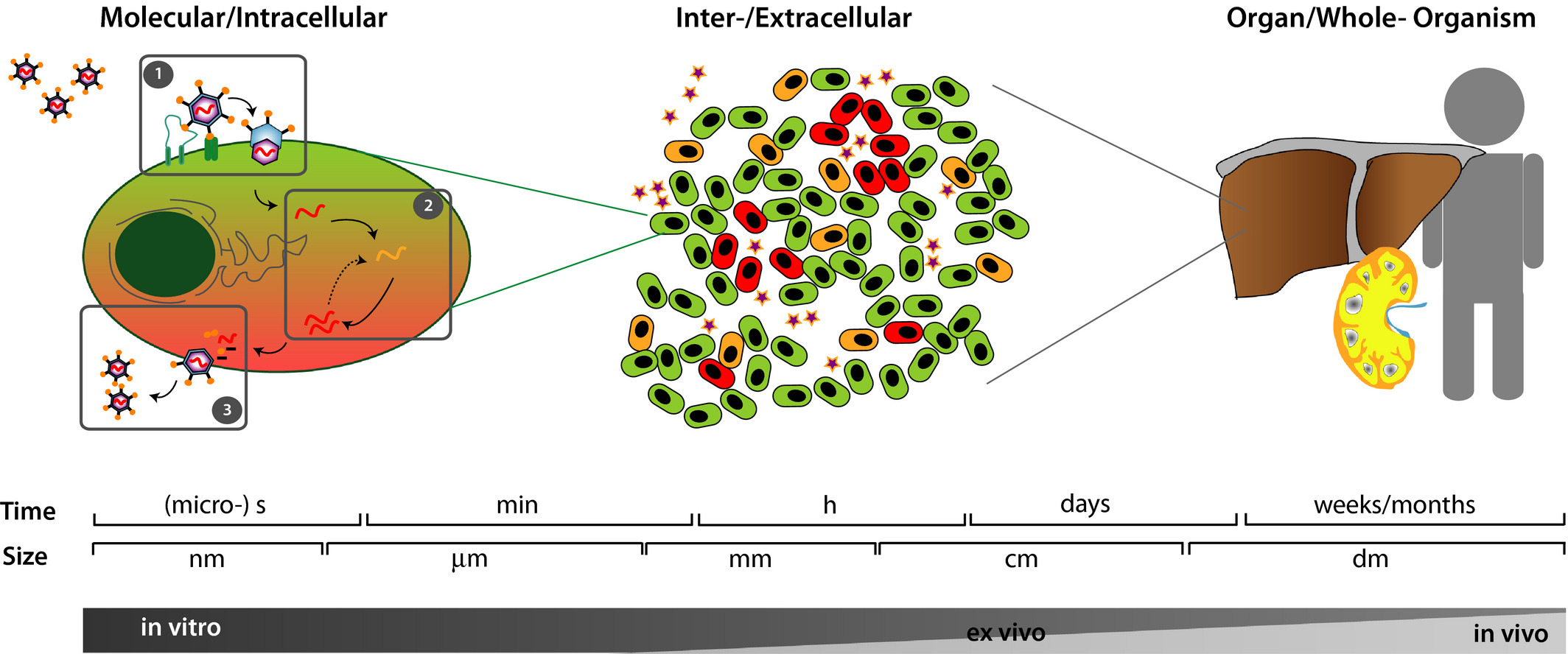
| Image: Viral infections are multiscale problems (Kumberger et al. FEBS Letters 2016). On the molecular and subcellular level (left), we are concerned with virus entry, replication and exit. For example, an essential question on this level is how exactly the virus gets across the cell membrane, with fusion and endocytosis being the most prominent pathways. On the level of cell populations (middle), we deal with viral spread from infected to susceptible cells. After being infected, cells first start to produce viruses and later are removed from the population (either becoming immune or dying). An essential question in this context is how exactly spread occurs (e.g. through cell-free virions or through direct cell-cell contacts). On the level of organisms (right), we are concerned with the infection of an organ, such as the liver for hepatitis viruses or the lung for SARS-CoV-2, and the spread between organs (e.g. SARS-CoV-2 might affect both lung and heart). Not shown is the level of population of individuals, for which models are similar to the ones for cell populations. Again the mode of spreading is key, e.g. droplet infection versus direct contact for SARS-CoV-2. |
Viruses have a very small genome (made either from DNA, like herpes, papilloma or hepatitis B viruses, or from RNA, like HIV or SARS-CoV-2) that is encapsulated by a protein shell (the viral capsid). Many of them are in addition wrapped by a lipid bilayer (enveloped viruses, all of the above mentioned except papilloma). Because they are not autonomous in regard to metabolism and replication, they need a host cell to proliferate and thus are not considered to be alive. Due to their simplicity compared with biological cells, they have always fascinated physicists, starting with the early work by Watson and Crick on the shape of viruses (Nature 1956) and the mathematical theory by Caspar and Klug 1962 on the crystallographic structure of virus capsids (Nobel prize 1982). Today we have a well-developed body of theory on the structure, mechanics and assembly of virus capsids. Moreover physicists are also very interested in the larger scale processes related to viruses, in particular on how they evolve and how they spread in populations of cells and individuals.
|
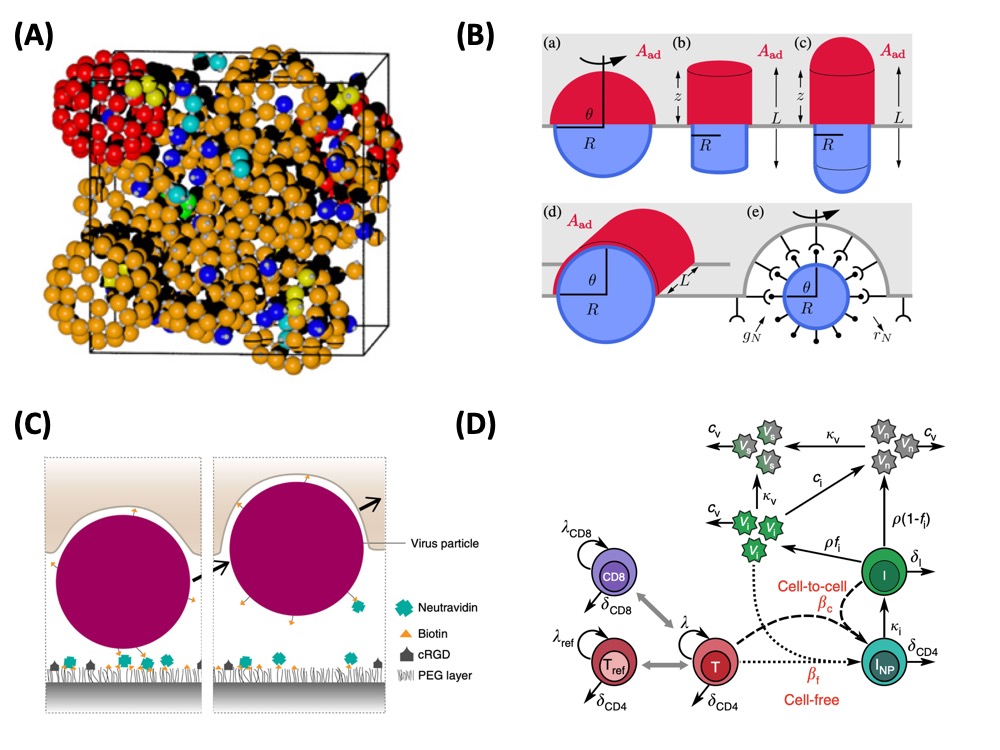
| Image: (A) Virus capsid assembly can be efficiently simulated with Brownian dynamics of patchy particles. We found that hierarchical assembly and specific ranges for inflow rates can speed up virus production (Baschek et al. BMC Biophysics 2012; Boettcher et al. Physical Biology 2015). (B) Virus uptake strongly depends on virus size and shape. In general, cylinders are taken up faster, but spheres can profit more from stochastic noise (Frey, Ziebert and Schwarz, PRL and PRE 2019). (C) Uptake of reovirus occurs on the ventral (or basal) side of adherent cells. We found that this process is mainly determined by the adhesion energy (Wiegand et al. Nature Communications 2020). (D) It has long been debated whether HIV-1 spreads predominantly by cell-free virions or by direct cell-cell contact. Combining long-term experiments with infected T-cells in collagen gels with mathematical modelling, we showed that cell-cell contact dominate, and that typical contacts last for about 20 min (Imle et al. Nature Communications 2019). |
In our group, we have worked on several theory aspects of the physics of viruses. Using Brownian dynamics of patchy particles, we have shown that the hierarchical assembly can be more efficient than direct one (Baschek et al. BMC Biophysics 2012) and that in an open system, the rate of monomer influx matters a lot (Boettcher et al. Physical Biology 2015). Regarding virus uptake at cell membranes, we have developed an analytical framework to calculate the speed of uptake as a function of size and shape (Frey, Ziebert and Schwarz, PRL and PRE 2019). We found that in general cylindrical viruses are taken up faster, but that spherical viruses can profit from stochastic noise for small system size. We then have calculated phase diagrams for uptake, e.g. as a function of membrane tension and line tension.
We also collaborate closely with experimental groups on virus uptake and spread, especially in the context of the DFG Collaborative Research Center 1129 on integrative analysis of pathogen replication and spread. With the group of Joachim Spatz, we studied the forces during uptake of reovirus and adhesive nanoparticles at the ventral membrane of adherent cells (Wiegand et al. Nature Communications 2020). With the group of Oliver Fackler, we studied the spread of HIV-1 in collagen gels (Imle et al. Nature Communications 2019). Using kinetic and Cellular Potts models, we found that direct cell-cell contact accounts for the infection, and we were able to estimate the typical contact time as 20 min, which seems to be required to transmit the virus from one cell to another. Interestingly, a dense collagen gel seems to help the cells to migrate and to establish contacts.
Review
- Peter Kumberger, Felix Frey, Ulrich S Schwarz, and Frederik Graw. Multiscale modeling of virus replication and spread. FEBS letters, 590: 1972-1986 (2016) (journal website, PDF)
Selected publications from collaborations with experimentalists
- Andrea Imle, Peter Kumberger, Nikolas D Schnellbaecher, Jana Fehr, Paola Carrillo-Bustamante, Janez Ales, Philip Schmidt, Christian Ritter, William J Godinez, Barbara Mueller, Karl Rohr, Fred A. Hamprecht, Ulrich S. Schwarz, Frederik Graw, and Oliver T. Fackler. Experimental and computational analyses reveal that environmental restrictions shape HIV-1 spread in 3d cultures. Nature communications, 10(1):2144, 2019. (journal website, PDF)
- Tina Wiegand, Marta Fratini, Felix Frey, Klaus Yserentant, Yang Liu, Eva Weber, Kornelia Galior, Julia Ohmes, Felix Braun, Dirk-Peter Herten, Steeve Boulant, Ulrich S. Schwarz, Khalid Salaita, E. Ada Cavalcanti-Adam and Joachim P. Spatz. Forces during cellular uptake of viruses and nanoparticles at the ventral side. Nature Communications, 11(1):1-13, 2020. (journal website, PDF)
Selected theory work on viruses
- Johanna E. Baschek, Heinrich C. R. Klein, and Ulrich S. Schwarz. Stochastic dynamics of virus capsid formation: Direct versus hierarchical self-assembly. BMC Biophysics, 5:22, 2012. (journal website, PDF, arXiv:1502.00156)
- Marvin A Boettcher, Heinrich C R Klein, and Ulrich S Schwarz. Role of dynamic capsomere supply for viral capsid self-assembly. Physical Biology, 12:016014, 2015. (journal website, PDF, arXiv)
- Felix Frey, Falko Ziebert, and Ulrich S Schwarz. Stochastic dynamics of nanoparticle and virus uptake. Physical Review Letters, 122(8):088102, 2019. (journal website, PDF, arXiv)
- Felix Frey, Falko Ziebert, and Ulrich S. Schwarz. Dynamics of particle uptake at cell membranes. Phys. Rev. E, 100:052403, Nov 2019. (journal website, arXiv)
Last modified Mar 24 2020 by USS.
Back to home page Ulrich Schwarz.